Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells
Abstract
1. Introduction
2. Results
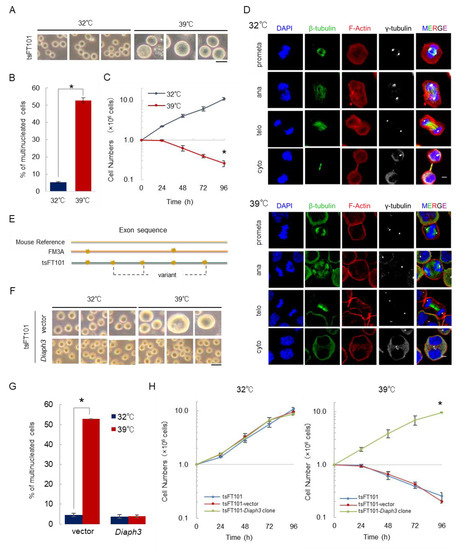
2.1. Diaph3 is the Gene Responsible for Temperature Sensitivity of tsFT101 Cells under High Temperature Conditions
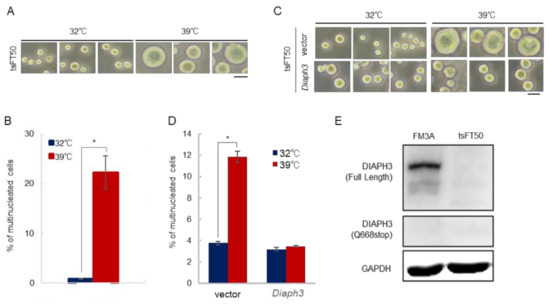
2.2. Diaph3 Is not Expressed at the Protein Level in tsFT50 Cells
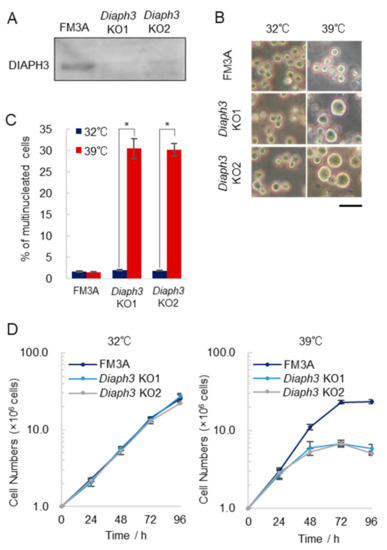
2.3. Diaph3 Knockout in FM3A Cells also Induces Temperature Sensitivity
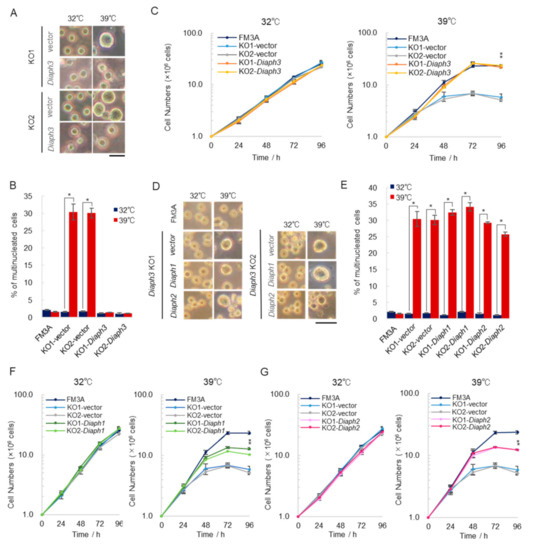
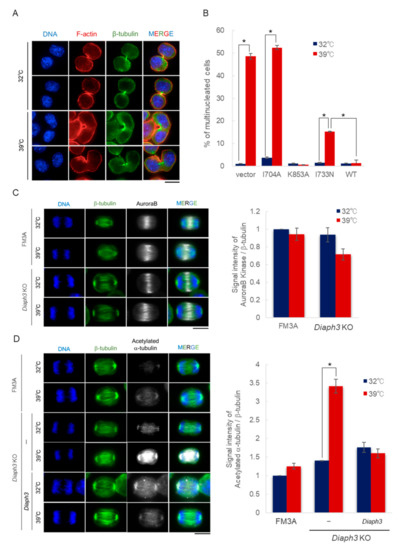
2.4. Overexpression of Diaph3, but Not Diaph1 and Diaph2, Rescues the Temperature Sensitivity Exhibited by Diaph3 KO Cells under High Temperature Conditions
2.5. Diaph3 Regulates Cytokinesis via Controlling the Stability of Microtuble at High Temperatures
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Multinucleation Analysis
4.3. Immunostaining
4.4. Exome Analysis
4.5. Cloning and Lentivirus Infection
4.6. Western Blot (WB) Analysis
4.7. Diaph3 Knockout by the CRISPR-Cas9 System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, V.; Mogilner, A.; Maresca, T.J. Classical and Emerging Regulatory Mechanisms of Cytokinesis in Animal Cells. Biology 2019, 8, 55. [Google Scholar] [CrossRef][Green Version]
- Pollard, T.D.; O’Shaughnessy, B. Molecular Mechanism of Cytokinesis. Annu. Rev. Biochem. 2019, 88, 661–689. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holland, A.J.; Cleveland, D.W. Losing balance: The origin and impact of aneuploidy in cancer. EMBO Rep. 2012, 13, 501–514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shiratsuchi, G.; Takaoka, K.; Ashikawa, T.; Hamada, H.; Kitagawa, D. RBM14 prevents assembly of centriolar protein complexes and maintains mitotic spindle integrity. EMBO J. 2015, 34, 97–114. [Google Scholar] [CrossRef][Green Version]
- Roper, R.J.; Reeves, R.H. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2006, 2, e50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maiburg, M.; Repping, S.; Giltay, J. The genetic origin of Klinefelter syndrome and its effect on spermatogenesis. Fertil. Steril. 2012, 98, 253–260. [Google Scholar] [CrossRef]
- O’Connell, K.F.; Leys, C.M.; White, J.G. A genetic screen for temperature-sensitive cell-division mutants of Caenorhabditis elegans. Genetics 1998, 149, 1303–1321. [Google Scholar]
- Murakami, Y.; Yasuda, H.; Miyazawa, H.; Hanaoka, F.; Yamada, M. Characterization of a temperature-sensitive mutant of mouse FM3A cells defective in DNA replication. Proc. Natl. Acad. Sci. USA 1985, 82, 1761–1765. [Google Scholar] [CrossRef][Green Version]
- Murakami, Y.; Eki, T.; Miyazawa, H.; Enomoto, T.; Hanaoka, F.; Yamada, M. Further characterization of a murine temperature-sensitive mutant, tsFT20 strain, containing heat-labile DNA polymerase alpha-activity. Exp. Cell Res. 1986, 163, 135–142. [Google Scholar] [CrossRef]
- Mineo, C.; Murakami, Y.; Ishimi, Y.; Hanaoka, F.; Yamada, M. Isolation and analysis of a mammalian temperature-sensitive mutant defective in G2 functions. Exp. Cell Res. 1986, 167, 53–62. [Google Scholar] [CrossRef]
- Eki, T.; Enomoto, T.; Miyajima, A.; Miyazawa, H.; Murakami, Y.; Hanaoka, F.; Yamada, M.; Ui, M. Isolation of temperature-sensitive cell cycle mutants from mouse FM3A cells. Characterization of mutants with special reference to DNA replication. J. Biol. Chem. 1990, 265, 26–33. [Google Scholar] [PubMed]
- Schönichen, A.; Geyer, M. Fifteen formins for an actin filament: A molecular view on the regulation of human formins. Biochim. Biophys. Acta 2010, 1803, 152–163. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartolini, F.; Moseley, J.B.; Schmoranzer, J.; Cassimeris, L.; Goode, B.L.; Gundersen, G.G. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J. Cell Biol. 2008, 181, 523–536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yasumitsu, H.; Hanaoka, F.; Yasuda, H.; Murakami, Y.; Enomoto, T.; Yamada, M. Isolation and initial characterization of a temperature-sensitive mutant of mouse FM3A cells defective in cytokinesis. Cell Struct. Funct. 1985, 10, 79–88. [Google Scholar] [CrossRef][Green Version]
- Ben-Aroya, S.; Pan, X.; Boeke, J.D.; Hieter, P. Making temperature-sensitive mutants. Methods Enzymol. 2010, 470, 181–204. [Google Scholar]
- Meiring, J.C.M.; Bryce, N.S.; Niño, J.L.G.; Gabriel, A.; Tay, S.S.; Hardeman, E.C.; Biro, M.; Gunning, P.W. Tropomyosin concentration but not formin nucleators mDia1 and mDia3 determines the level of tropomyosin incorporation into actin filaments. Sci. Rep. 2019, 9, 6504. [Google Scholar] [CrossRef]
- Watanabe, S.; Ando, Y.; Yasuda, S.; Hosoya, H.; Watanabe, N.; Ishizaki, T.; Narumiya, S. mDia2 Induces the Actin Scaffold for the Contractile Ring and Stabilizes Its Position during Cytokinesis in NIH 3T3 Cells. Mol. Biol. Cell 2008, 19, 2328–2338. [Google Scholar] [CrossRef][Green Version]
- Kamijo, K.; Ohara, N.; Abe, M.; Uchimura, T.; Hosoya, H.; Lee, J.-S.; Miki, T. Dissecting the Role of Rho-mediated Signaling in Contractile Ring Formation. Mol. Biol. Cell 2006, 17, 43–55. [Google Scholar] [CrossRef][Green Version]
- Glotzer, M. The Molecular Requirements for Cytokinesis. Science 2005, 307, 1735–1739. [Google Scholar] [CrossRef][Green Version]
- Gruneberg, U.; Neef, R.D.; Honda, R.; Nigg, E.A.; Barr, F.A. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 2004, 166, 167–172. [Google Scholar] [CrossRef][Green Version]
- Guse, A.; Mishima, M.; Glotzer, M. Phosphorylation of ZEN-4/MKLP1 by aurora B regulates completion of cytokinesis. Curr. Biol. 2005, 15, 778–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Barrera, J.; Bernabé-Rubio, M.; Casares-Arias, J.; Rangel, L.; Fernández-Martín, L.; Correas, I.; Alonso, M.A. The actin-MRTF-SRF transcriptional circuit controls tubulin acetylation via α-TAT1 gene expression. J. Cell Biol. 2018, 217, 929–944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, S.; Sterling, N.A.; Junker, I.P.; Helm, C.T.; Smith, G.M. Effects of αTAT1 and HDAC5 on axonal regeneration in adult neurons. PLoS ONE 2017, 12, e0177496. [Google Scholar] [CrossRef] [PubMed]
- Ramabhadran, V.; Gurel, P.S.; Higgs, H.N. Mutations to the formin homology 2 domain of INF2 protein have unexpected effects on actin polymerization and severing. J. Biol. Chem. 2012, 287, 34234–34245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thurston, S.F.; Kulacz, W.A.; Shaikh, S.; Lee, J.M.; Copeland, J.W. The Ability to Induce Microtubule Acetylation Is a General Feature of Formin Proteins. PLoS ONE 2012, 7, e48041. [Google Scholar] [CrossRef] [PubMed]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.-F.; Yao, T.-P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Grueb, S.S.; Muhs, S.; Popp, Y.; Schmitt, S.; Geyer, M.; Lin, Y.N.; Windhorst, S. The formin Drosophila homologue of Diaphanous2 (Diaph2) controls microtubule dynamics in colorectal cancer cells independent of its FH2-domain. Sci. Rep. 2019, 9, 5352. [Google Scholar] [CrossRef]
- Watanabe, S.; Okawa, K.; Miki, T.; Sakamoto, S.; Morinaga, T.; Segawa, K.; Arakawa, T.; Kinoshita, M.; Ishizaki, T.; Narumiya, S. Rho and anillin-dependent control of mDia2 localization and function in cytokinesis. Mol. Biol. Cell 2010, 21, 3193–3204. [Google Scholar] [CrossRef][Green Version]
- Gaillard, J.; Ramabhadran, V.; Neumanne, E.; Gurel, P.; Blanchoin, L.; Vantard, M.; Higgs, H.N. Differential interactions of the formins INF2, mDia1, and mDia2 with microtubules. Mol. Biol. Cell 2011, 22, 4575–4587. [Google Scholar] [CrossRef]
- Shin, H.; Song, H.; Suh, C.S.; Lim, H.J. The formin protein mDia2 serves as a marker of spindle pole dynamics in vitrified-warmed mouse oocytes. PLoS ONE 2013, 8, e75729. [Google Scholar] [CrossRef][Green Version]
- Kim, H.C.; Jo, Y.J.; Kim, N.H.; Namgoong, S. Small molecule inhibitor of formin homology 2 domains (SMIFH2) reveals the roles of the formin family of proteins in spindle assembly and asymmetric division in mouse oocytes. PLoS ONE 2015, 10, e0123438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Damiani, D.; Goffinet, A.M.; Alberts, A.; Tissir, F. Lack of Diaph3 relaxes the spindle checkpoint causing the loss of neural progenitors. Nat. Commun. 2016, 7, 13509. [Google Scholar] [CrossRef] [PubMed]
- Weisenberg, R.C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science 1972, 177, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Brown, D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J. Am. Soc. Nephrol. 1998, 9, 155–166. [Google Scholar]
- Li, G.; Moore, J.K. Microtubule dynamics at low temperature: Evidence that tubulin recycling limits assembly. Mol. Biol. Cell 2020, 31, 1154–1166. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, J.; Ahmad, S.; Rozier, L.; Yu, H.; Deng, H.; Mao, Y. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev. Cell 2011, 20, 342–352. [Google Scholar] [CrossRef][Green Version]
- Pechan, P.M. Heat shock proteins and cell proliferation. FEBS Lett. 1991, 280, 1–4. [Google Scholar] [CrossRef][Green Version]
- Huang, L.; Mivechi, N.F.; Moskophidis, D. Insights into regulation and function of the major stress-induced hsp70 molecular chaperone in vivo: Analysis of mice with targeted gene disruption of the hsp70.1 or hsp70.3 gene. Mol. Cell. Biol. 2001, 21, 8575–8591. [Google Scholar] [CrossRef][Green Version]
- Radons, J. The human HSP70 family of chaperones: Where do we stand? Cell Stress Chaperones 2016, 21, 379–404. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Stokes, J., 3rd; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef][Green Version]
- Vidair, C.A.; Doxsey, S.J.; Dewey, W.C. Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J. Cell. Physiol. 1993, 154, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, K.; Miyakoda, M.; Suzuki, K.; Kodama, S.; Watanabe, M. Heat shock induces centrosomal dysfunction, and causes non-apoptotic mitotic catastrophe in human tumour cells. Int. J. Hyperth. 2002, 18, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Hut, H.M.; Kampinga, H.H.; Sibon, O.C. Hsp70 protects mitotic cells against heat-induced centrosome damage and division abnormalities. Mol. Biol. Cell 2005, 16, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Srinivas, U.K. Heat shock induces chromosomal instability in near-tetraploid embryonal carcinoma cells. Cancer Biol. Ther. 2008, 7, 1471–1480. [Google Scholar] [CrossRef][Green Version]
- Vertii, A.; Zimmerman, W.; Ivshina, M.; Doxsey, S. Centrosome-intrinsic mechanisms modulate centrosome integrity during fever. Mol. Biol. Cell 2015, 26, 3451–3463. [Google Scholar] [CrossRef]
- O’Regan, L.; Sampson, J.; Richards, M.W.; Knebel, A.; Roth, D.; Hood, F.E.; Straube, A.; Royle, S.J.; Bayliss, R.; Fry, A.M. Hsp72 is targeted to the mitotic spindle by Nek6 to promote K-fiber assembly and mitotic progression. J. Cell Biol. 2015, 209, 349–358. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Morley, S.; Le, M.; Bedoret, D.; Umetsu, D.T.; Di Vizio, D.; Freeman, M.R. Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells: Potential effects on the tumor microenvironment. Cancer Biol. Ther. 2014, 15, 409–418. [Google Scholar] [CrossRef][Green Version]
- Dong, L.; Li, Z.; Xue, L.; Li, G.; Zhang, C.; Cai, Z.; Li, H.; Guo, R. DIAPH3 promoted the growth, migration and metastasis of hepatocellular carcinoma cells by activating beta-catenin/TCF signaling. Mol. Cell. Biochem. 2018, 438, 183–190. [Google Scholar] [CrossRef]
- Dvorak, K.M.; Pettee, K.M.; Rubinic-Minotti, K.; Su, R.; Nestor-Kalinoski, A.; Eisenmann, K.M. Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2). PLoS ONE 2018, 13, e0195278. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazama, H.; Kashiwaba, S.-i.; Ishii, S.; Yoshida, K.; Yatsuo, Y.; Naraoka, T.; Fukuoka, M.; Murakami, Y. Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells. Int. J. Mol. Sci. 2020, 21, 8493. https://doi.org/10.3390/ijms21228493
Kazama H, Kashiwaba S-i, Ishii S, Yoshida K, Yatsuo Y, Naraoka T, Fukuoka M, Murakami Y. Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells. International Journal of Molecular Sciences. 2020; 21(22):8493. https://doi.org/10.3390/ijms21228493
Chicago/Turabian StyleKazama, Hiroki, Shu-ichiro Kashiwaba, Sayaka Ishii, Keiko Yoshida, Yuta Yatsuo, Takuma Naraoka, Masashi Fukuoka, and Yasufumi Murakami. 2020. "Loss of DIAPH3, a Formin Family Protein, Leads to Cytokinetic Failure Only under High Temperature Conditions in Mouse FM3A Cells" International Journal of Molecular Sciences 21, no. 22: 8493. https://doi.org/10.3390/ijms21228493



No comments:
Post a Comment